Researchers from the National Institutes of Health have developed a three-dimensional structure that enables them to see how and where disease mutations on the twinkle protein can result in mitochondrial diseases. The protein is involved in helping cells use energy our bodies convert from food. Prior to the event of this 3D structure, researchers only had models and were unable to find out how these mutations contribute to disease. Mitochondrial diseases are a bunch of inherited conditions that affect 1 in 5,000 people and have only a few treatments.
For the primary time, we are able to map the mutations which are causing quite a few these devastating diseases. Clinicians can now see where these mutations lie and might use this information to assist pinpoint causes and help families make decisions, including decisions about having more children.”
Amanda A. Riccio, Ph.D., lead writer, researcher within the National Institute of Environmental Health Sciences (NIEHS) Mitochondrial DNA Replication Group
The brand new findings shall be particularly relevant for developing targeted treatments for patients that suffer from mitochondrial diseases equivalent to progressive external ophthalmoplegia, a condition that may result in lack of muscle functions involved in eye and eyelid movement; Perrault syndrome, a rare genetic disorder that could cause hearing loss; infantile-onset spinocerebellar ataxia, a hereditary neurological disorder; and hepatocerebral mitochondrial DNA (mtDNA) depletion syndrome, a hereditary disease that may result in liver failure and neurological complications during infancy.
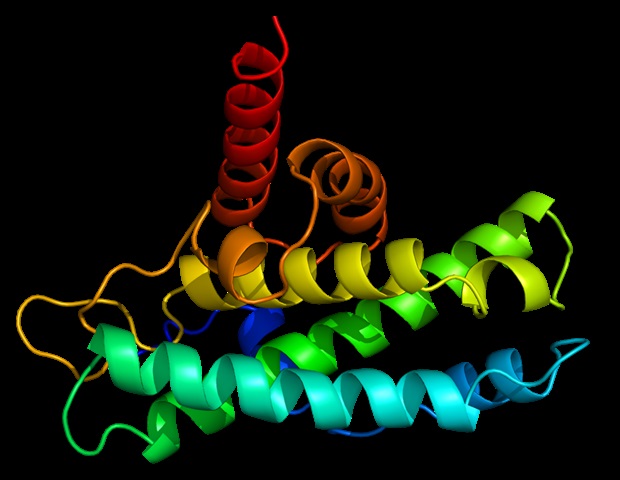
The paper that appears within the Proceedings of the National Academy of Sciences showcases how the NIEHS researchers were the primary to accurately map clinically relevant variants within the twinkle helicase, the enzyme that unwinds the mitochondrial DNA double helix. The twinkle structure and all of the coordinates are actually available within the open data Protein Data Bank that’s freely available to all researchers.
“The structure of twinkle has eluded researchers for a few years. It’s a really difficult protein to work with,” noted William C. Copeland, Ph.D., who leads the Mitochondrial DNA Replication Group and is the corresponding writer on the paper. “By stabilizing the protein and using the very best equipment on the earth we were in a position to construct the last missing piece for the human mitochondrial DNA replisome.”
The researchers used cryo-electron microscopy (CryoEM), which allowed them to see contained in the protein and the intricate structures of a whole bunch of amino acids or residues and the way they interact.
Mitochondria, that are answerable for energy production, are especially vulnerable to mutations. mtDNA mutations can disrupt their ability to generate energy efficiently for the cell. Unlike other specialized structures in cells, mitochondria have their very own DNA. In a cell’s nucleus there are two copies of every chromosome, nonetheless within the mitochondria there could possibly be 1000’s of copies of mtDNA. Having a high variety of mitochondrial chromosomes allows the cell to tolerate a number of mutations, but accumulation of too many mutated copies results in mitochondrial disease.
To conduct the study, the researchers used a clinical mutation, W315L, known to cause progressive external ophthalmoplegia, to unravel the structure. Using CryoEM, they were in a position to observe 1000’s of protein particles appearing in numerous orientations. The ultimate structure shows a multi-protein circular arrangement. Additionally they used mass spectrometry to confirm the structure after which did computer simulations to grasp why the mutation leads to disease.
Inside twinkle, they were in a position to map as much as 25 disease-causing mutations. They found that a lot of these disease mutations map right on the junction of two protein subunits, suggesting that mutations on this region would weaken how the subunits interact and make the helicase unable to operate.
“The arrangement of twinkle is loads like a puzzle. A clinical mutation can change the form of the twinkle pieces, and so they may now not fit together properly to perform the intended function,” Riccio explained.
“What’s so beautiful about Dr. Riccio and the team’s work is that the structure means that you can see so a lot of these disease mutations assembled in a single place,” said Matthew J. Longley, Ph.D., an writer and NIEHS researcher. “It is extremely unusual to see one paper that explains so many clinical mutations. Due to this work, we’re one step closer to having information that will be used to develop treatments for these debilitating diseases.”
Source:
National Institutes of Health
Journal reference:
Riccio, A.A., et al. (2022) Structural insight and characterization of human Twinkle helicase in mitochondrial disease. PNAS. doi.org/10.1073/pnas.2207459119.