In a recent study published within the journal JAMA Network, researchers in the USA investigated aspects that restricted the usage of antivirals directed at severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) in the USA (US) between January and February 2022.
Study: Early Adoption of Anti–SARS-CoV-2 Pharmacotherapies Amongst US Veterans With Mild to Moderate COVID-19, January and February 2022. Image Credit: Panaceum Media / Shutterstock
Background
Within the US, the incidence of coronavirus disease 2019 (COVID-19) was at its highest in January 2022. Older adults with preexisting comorbidities, equivalent to obesity, chronic kidney disease, and obesity, have remained at increased risk for severe COVID-19 for the reason that pandemic began. Hence, the authors selected the Veterans Affairs (VA) data, the most important integrated healthcare system within the US, to guage aspects related to the receipt of outpatient COVID-19 pharmacotherapies.
Several pharmacotherapies received emergency use authorization (EUA) for treating individuals with mild to moderate COVID-19 who were at high risk for progression to severe disease within the US during this time. For example, nirmatrelvir boosted with ritonavir and molnupiravir received EUA in late December 2021 and remdesivir for outpatient use in January 2022. Nevertheless, their supply for COVID-19 outpatients remained limited for some time, leading to an underuse of prescriptions for these pharmacotherapies.
Also, there will need to have been logistical barriers to pharmacotherapy administration and a lack of expertise amongst clinicians and the general public about these therapeutic options. Clinicians also probably failed to acknowledge the symptomatic disease early and link it to the available treatment.
In regards to the study
In the current study, researchers enrolled 111,717 outpatient US veterans with clinical risk aspects for severe COVID-19 who tested positive for SARS-CoV-2 between January and February 2022. The authors primarily focused on monitoring prescriptions of 4 COVID-19 pharmacotherapies viz., sotrovimab, a monoclonal antibody with high neutralizing activity against Omicron, and antivirals nirmatrelvir, molnupiravir, and remdesivir.
The team estimated the percentages of receipt of any of the 4 pharmacotherapies versus no treatment. The study used multivariable logistic regression models adjusted for age, gender, race, and ethnicity. Amongst veterans who received treatment, additionally they described the relative proportion of every of the 4 pharmacotherapies prescribed by the Veterans Integrated Services Network (VISN). Moreover, they monitored the study population for demographic characteristics, place of residence, underlying medical conditions, and COVID-19 vaccination status.
Study findings
Unfortunately, the proportion of people at high risk for progression to severe COVID-19 was high amongst untreated veterans during this early period. The researchers observed that hardly 3.8% received any outpatient COVID-19 pharmacotherapy. Of those, 5.5% had COVID-19–related symptoms, while 3.4% were unvaccinated or only partially vaccinated. Around two-thirds of veterans participating on this study were fully vaccinated or boosted. Nevertheless, partially vaccinated veterans were more prone to be treated, suggesting that veterans willing to hunt vaccination were also more likely to just accept really useful pharmacotherapies.
As expected, initial uptake of those treatments was low because oral antivirals were authorized just before the beginning of the study period. Nevertheless, it regularly greater than doubled from 3.1% in January to 7.1% in February 2022. Later, as drugs attained a comparatively surplus supply, their low usage amongst veterans of the VA reflected complex barriers to optimal use observed within the US and plenty of other countries.
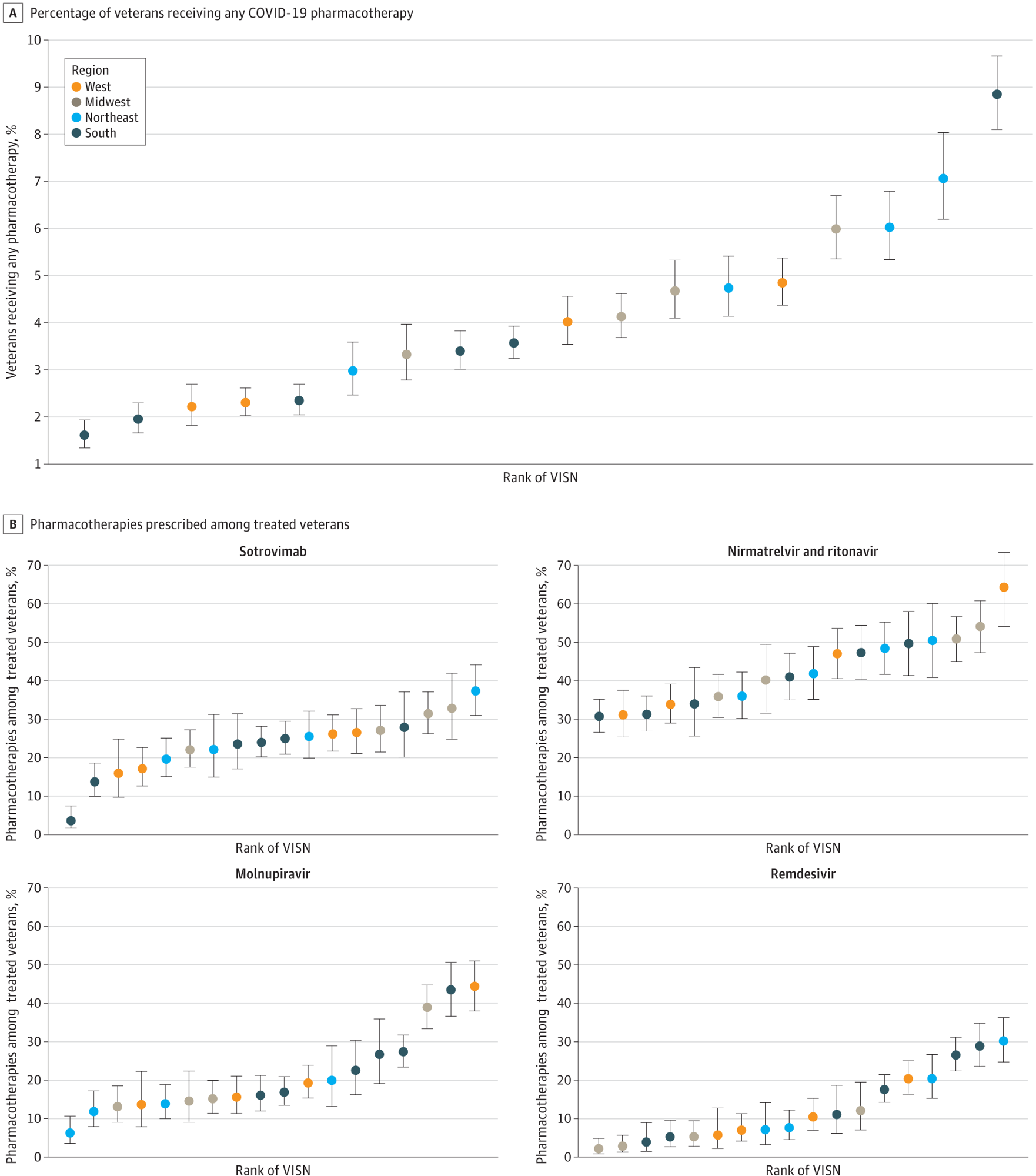 A, Percentage of veterans receiving any COVID-19 pharmacotherapy. B, Pharmacotherapies prescribed amongst treated veterans. Regions are based on VISNs. West includes VISNs 19 to 22; Midwest: 10, 12, 15, 23; Northeast: 1, 2, 4, 5; South: 6 to 9, 16, 17. Error bars indicate 95% CIs for proportions; VISN, Veterans Integrated Services Network.
A, Percentage of veterans receiving any COVID-19 pharmacotherapy. B, Pharmacotherapies prescribed amongst treated veterans. Regions are based on VISNs. West includes VISNs 19 to 22; Midwest: 10, 12, 15, 23; Northeast: 1, 2, 4, 5; South: 6 to 9, 16, 17. Error bars indicate 95% CIs for proportions; VISN, Veterans Integrated Services Network.
Although older veterans with more preexisting medical conditions were more prone to receive treatment, those from the Racial disparities in COVID-19–related care inside the VA system have been less pronounced. Yet, study results identified that individuals from the Black and Hispanic ethnicities were less prone to receive treatment. Possible reasons could possibly be their lower trust within the healthcare system or potential clinician biases.
The authors also noted marked differences within the distribution of those 4 COVID-19 pharmacotherapies based on geographical location. Accordingly, the authors observed a better likelihood of receiving treatment amongst rural US veterans, most of whom live in rural settings. Physical distance from VA facilities didn’t look like a considerable barrier to treatment.
Clinical practice varies across VISNs; subsequently, there have been notable differences within the relative use of various pharmacotherapies across VISNs. This finding also reflected differences in local infrastructure and clinician education. In some localities during this early period when the US government had not launched the nationwide COVID-19 Test to Treat Initiative, public opinion was skewed in regards to the authorized COVID-19 therapies within the absence of clinical trials or observational study evidence.
Conclusions
The present study findings further reinstated that the low use of COVID-19 pharmacotherapies during this early period post their authorization was not unique to US veterans. Studies have made similar observations across different populations and settings. For example, studies have noted low use of COVID-19 monoclonal antibody treatments in non–veteran populations of minority racial and ethnic groups.
As more observational studies and clinical trials will gather more data establishing the effectiveness of currently used COVID-19 pharmacotherapies, improved infrastructure, and patient education, their usage might improve. Data showing the comparative effectiveness of those pharmacotherapies may even be critical to informing clinical practices.