In a recent study published within the journal Nature Communications, researchers investigated whether coronavirus disease 2019 (COVID-19) messenger ribonucleic acid (mRNA) vaccines induce long-term resident memory T cells within the lungs against severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2).
Study: Limited induction of polyfunctional lung-resident memory T cells against SARS-CoV-2 by mRNA vaccination in comparison with infection. Image Credit: piccreative / Shutterstock
Background
While COVID-19 vaccines developed after the onset of the pandemic have successfully reduced SARS-CoV-2 infections, the extent of protection these vaccines stays unclear. Despite declining vaccine efficacy over time and latest variants eliciting humoral immunity, neutralizing antibodies still exhibit significant cross-protection against COVID-19.
Moreover, the cellular immunity induced by SARS-CoV-2 infections has been detected in peripheral blood and as resident memory T cells within the lungs, with the variety of SARS-CoV-2-specific resident memory T cells correlating to the extent of protection.
Recent research indicates that the monovalent mRNA COVID-19 vaccines, resembling mRNA-1273 and BNT162b2 produced by Moderna and Pfizer/BioNTech, respectively, induce significant levels of CD8+ and CD4+ T-cell responses in peripheral blood, and the interferon-gamma (IFNγ) responses against the SARS-CoV-2 spike protein increase after booster doses of the mRNA vaccine. Nonetheless, whether these mRNA vaccines elicit long-lasting SARS-CoV-2-specific memory T cells that reside within the lungs stays to be explored.
In regards to the study
In the current study, the researchers compared paired lung cross-sections and peripheral blood samples from 4 cohorts — individuals that were unvaccinated but were also uninfected, unvaccinated individuals who were under long-term SARS-CoV-2 convalescence, individuals who had received two or three doses of the mRNA vaccine in the long run but had not been infected, and uninfected individuals who had received three or 4 doses of the mRNA vaccine within the short term. The lung cross-sections were obtained when the participants underwent lung resections for unrelated reasons, resembling suspected malignancies.
Peptide pools consisting of the SARS-CoV-2 membrane, spike, and nucleotide proteins were used to stimulate the cellular suspensions derived from the lung tissue and the peripheral blood mononuclear cells (PBMC) obtained from the participants to look at the cellular immune responses resembling those of interleukins (IL)-10, IL-4, and IFNγ. The degrees of SARS-CoV-2 spike-protein-specific T cells in lung and peripheral blood samples were also compared.
Chemoattraction assays using chemokine ligands (CCL) 19 and 21 and sphingosine-1-phosphate (S1P) were used to draw the SARS-CoV-2 membrane, spike, and nucleotide-specific T cells within the lung tissue to evaluate the character of the antigen-specific resident T cells within the lungs. The flexibility of the mRNA vaccine to induce polyfunctional SARS-CoV-2 spike-specific T-cell responses within the lungs and blood was also examined.
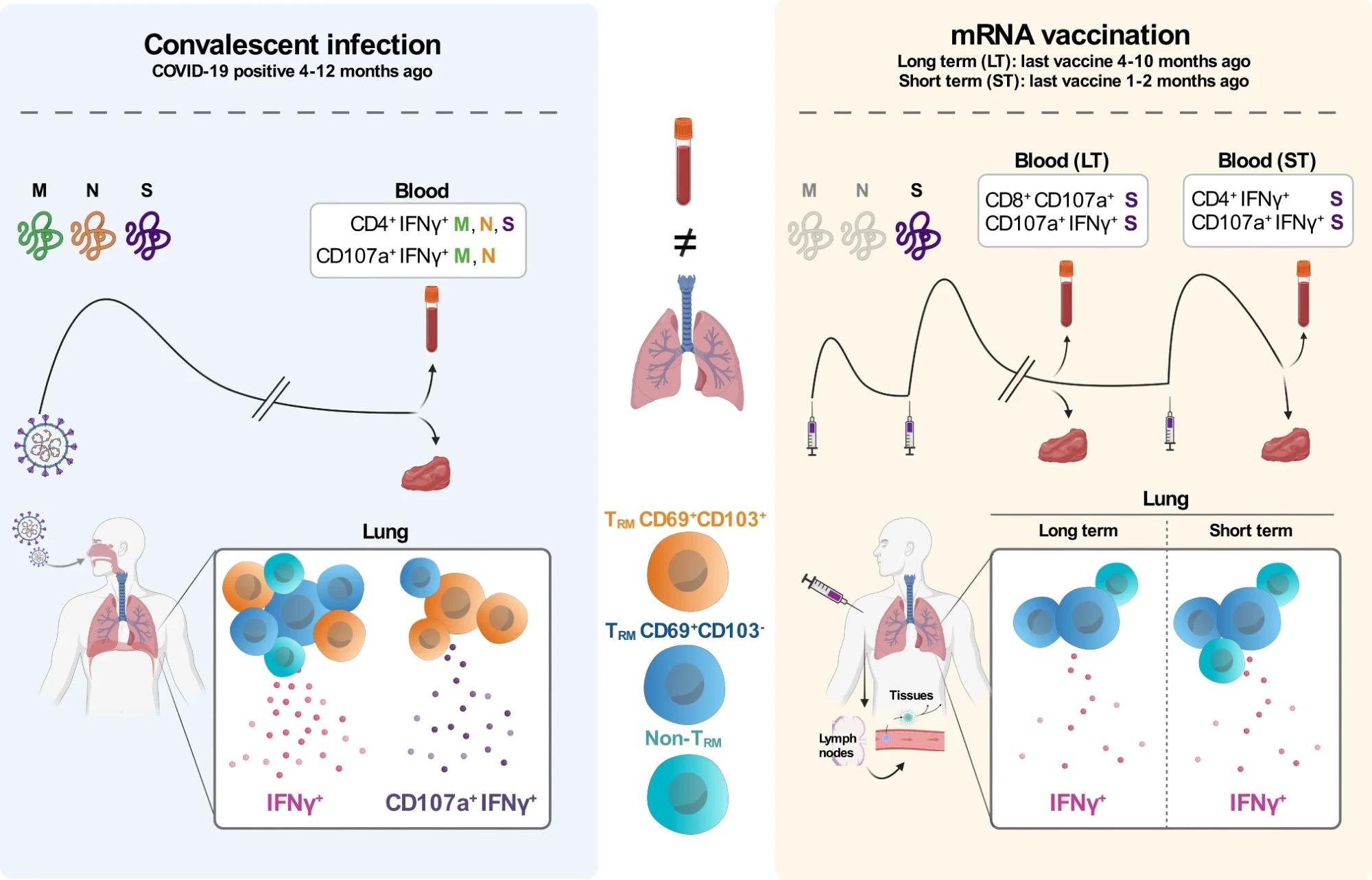 The main target of the study was to discover T cell responses against SARS-CoV-2 in paired blood and lung tissue samples of patients that recovered from COVID-19 between 4 to 12 months ago (convalescent infection, left panel) and patients that were uninfected and mRNA vaccinated either between 4 to 10 months ago (LT long run, right panel) or between 1 and a pair of months ago (ST short term, right panel). Left: Infection with SARS-CoV-2 triggers the immune system to reply against multiple viral proteins, including the M, N, and S proteins. Within the blood of convalescent patients, CD4+ T cells responded to M, N, and S peptides by producing IFNγ and each CD4+ and CD8+ T cells responding to M and N peptides by producing IFNγ and CD107a. Furthermore, within the lung of those patients, we found T cells with mainly TRM (CD69+CD103+ and CD69+CD103-) phenotypes producing IFNγ or a mix of IFNγ and CD107a. Right: mRNA-vaccinated patients are only exposed to mRNA encoding for the SARS-CoV-2 S protein. Within the blood of LT vaccinated patients, CD8+ T cells responded to S peptides by producing CD107a and CD4+ and CD8+ producing IFNγ and CD107a. Within the blood of ST-vaccinated patients, the response to S peptides mainly consisted of CD4+ T cells producing IFNγ and CD4+ and CD8+ producing IFNγ and CD107a. In contrast to T cell responses within the lung of convalescent-infected patients, each LT and ST mRNA-vaccinated patients showed a less distinguished IFNγ response mainly produced by CD69+CD103- TRM cells and non-TRM cells. Last, CD69+CD103+ TRM producing IFNγ and CD107a were virtually absent within the lungs of those vaccinated patients.
The main target of the study was to discover T cell responses against SARS-CoV-2 in paired blood and lung tissue samples of patients that recovered from COVID-19 between 4 to 12 months ago (convalescent infection, left panel) and patients that were uninfected and mRNA vaccinated either between 4 to 10 months ago (LT long run, right panel) or between 1 and a pair of months ago (ST short term, right panel). Left: Infection with SARS-CoV-2 triggers the immune system to reply against multiple viral proteins, including the M, N, and S proteins. Within the blood of convalescent patients, CD4+ T cells responded to M, N, and S peptides by producing IFNγ and each CD4+ and CD8+ T cells responding to M and N peptides by producing IFNγ and CD107a. Furthermore, within the lung of those patients, we found T cells with mainly TRM (CD69+CD103+ and CD69+CD103-) phenotypes producing IFNγ or a mix of IFNγ and CD107a. Right: mRNA-vaccinated patients are only exposed to mRNA encoding for the SARS-CoV-2 S protein. Within the blood of LT vaccinated patients, CD8+ T cells responded to S peptides by producing CD107a and CD4+ and CD8+ producing IFNγ and CD107a. Within the blood of ST-vaccinated patients, the response to S peptides mainly consisted of CD4+ T cells producing IFNγ and CD4+ and CD8+ producing IFNγ and CD107a. In contrast to T cell responses within the lung of convalescent-infected patients, each LT and ST mRNA-vaccinated patients showed a less distinguished IFNγ response mainly produced by CD69+CD103- TRM cells and non-TRM cells. Last, CD69+CD103+ TRM producing IFNγ and CD107a were virtually absent within the lungs of those vaccinated patients.
Results
The outcomes indicated that while the responses of the IFNγ-secreting CD4+ T cells against the SARS-CoV-2 spike protein were similar within the lung samples obtained from convalescing individuals and individuals immunized with the mRNA vaccine, the resident memory T cell phenotype presented less steadily within the lung responses from the vaccinated individuals as in comparison with those of the convalescing individuals. Moreover, the polyfunctional resident memory T cells exhibiting IFNγ and granulation marker CD107a were almost absent within the long-term and short-term vaccinated individuals.
The cellular immunity induced by symptomatic SARS-CoV-2 infection also seems stronger for the reason that magnitude of immune responses against the SARS-CoV-2 membrane and nucleotide proteins was higher than that against the spike protein. Nonetheless, the study did detect a rise within the responses of the IFNγ-secreting T cells after recent booster doses of the mRNA vaccine and enhanced IFNγ+ T cells within the lungs of short-term vaccinated individuals as in comparison with long-term vaccinated individuals.
The researchers imagine that the incorporation of other SARS-CoV-2 peptide fragments, resembling those of the nucleocapsid protein, and using mucosal delivery in future vaccines could establish optimal resident memory T cells within the lungs.
Conclusions
Overall, the outcomes indicated that mRNA vaccines don’t elicit comparable resident memory T cell responses or polyfunctional resident memory T cell responses against SARS-CoV-2 within the lungs as SARS-CoV-2 infections. Strategies for future vaccines could consider mucosal delivery routes and the incorporation of other SARS-CoV-2 proteins to make sure simpler memory T-cell responses.
Journal reference:
- Pieren, D. K. J., Kuguel, S. G., Rosado, J., Robles, A. G., Rey-Cano, J., Mancebo, C., Esperalba, J., Falcó, V., Buzón, M. J., & Genescà, M. (2023). Limited induction of polyfunctional lung-resident memory T cells against SARS-CoV-2 by mRNA vaccination in comparison with infection. Nature Communications, 14(1). https://doi.org/10.1038/s41467-023-37559-w, https://www.nature.com/articles/s41467-023-37559-w