What challenges do researchers normally encounter with conventional proteomic methods?
The proteomics field is extremely complex, spanning initial biological samples to comprehensive evaluation. An overarching theme repeatedly emphasized by researchers revolves across the absence of a universal solution for this whole process. Slightly, each phase demands meticulous harmonization.
Researchers often find themselves at a crossroads. They need to choose from targeted approaches, similar to arrays, immunoassays, or gels, where the search is predefined, or go for an unbiased technology that permits in-depth sample interrogation.
Attaining peptide-level resolution predominantly involves mass spectrometry — a costly avenue, with machines exceeding a million-dollar price tag, accompanied by demanding staffing and infrastructure prerequisites that not all institutions can accommodate.
How does next-generation protein sequencing provide a novel solution for these challenges?
In considering this query, it is crucial to acknowledge two distinct customer groups, each uniquely impacted by the benefits of next-generation protein sequencing.
For somebody dedicated to overseeing a core lab inside a well-funded academic research institute, catering to advanced technologies, including mass spectrometry and others, I believe this technology allows experts to delve deeper into their sample analyses.
While initial screening reveals the composition of the samples, next-generation protein sequencing technology facilitates a comprehensive interrogation of amino acid variations, modifications, and complex changes that solely sequencing can unveil, scrutinizing peptides on the amino acid level.
Conversely, there are also some researchers who may lack the extensive infrastructure or financial means to obtain costly mass spectrometry equipment. Presently, they outsource their sample analyses to core labs.
That is where the pivotal aspect of accessibility comes into play. Our proprietary machine, available at a reasonable cost of $70,000, grants them the autonomy to conduct testing inside their laboratories.
The mixing of automated evaluation negates the need for specialised personnel expert in tasks like bioinformatics. For this group, the main focus shifts towards internalizing and maintaining control over their research process, reducing reliance on external core labs.
Are you able to explain how the Platinum™ Next-Generation Protein Sequencer makes protein sequencing more accessible to researchers from various scientific backgrounds?
Platinum™ Next-Generation Protein Sequencer achieves this through two key avenues. Firstly, it addresses the financial aspect. No matter whether an establishment is large or small, acquiring such equipment demands a budget allocation.
Priced at $70,000, this instrument removes the need of securing substantial grants solely to access this technology. Researchers can more easily discover resources to make this technology investment, facilitating its integration into their research endeavors.
Secondly, the benefit of knowledge evaluation is outstanding. While certain analyses, similar to protein identification, might be performed on each our machine and a mass spectrometer, our platform distinguishes itself through automated post-analysis procedures.
In contrast, with a mass spectrometer, you either must have the staff to assist conduct this evaluation, or you would need to spend the time to try this evaluation yourself. Platinum™ Next-Generation Protein Sequencer not only ensures simplicity but in addition alleviates the onus on specialized staff, making routine tasks like protein identification more efficient.
How does the Platinum sequencer provide high-resolution protein sequence data without requiring extensive expertise or complex infrastructure, and why is that this vital?
The basic principle underlying this technological approach is the utilization of normal sample preparation methods commonly employed in proteomics laboratories.
These labs typically utilize techniques similar to immuoprecipitation or sample depletion methods. The revolutionary aspect of next-generation protein sequencing, which drives enhanced insights, revolves across the breakdown of proteins into quite a few individual peptides.
This technology employs a mix of specialised sequencing reagents, a semiconductor chip, and a dedicated machine. This mix facilitates a careful process wherein the peptide is sequentially cleaved, one amino acid at a time, and accurately measured.
Consequently, this procedure’s intricacy and labor-intensive nature, which might conventionally demand significant resources inside a laboratory setting, are efficiently condensed inside our proprietary machine and technology. The culmination of this process yields invaluable data.
Figure 1. Platinum’s easy-to-use, end-to-end solution includes every part you might want to prepare, sequence and analyze proteins with seamless integration into existing workflows. The Platinum workflow begins with protein digestion and immobilization of peptides on a semiconductor chip. Fluorescently labeled N-terminal amino acid (NAA) recognizers bind each NAA and the binding intensity and kinetics are captured as a novel kinetic signature for every NAA. Aminopeptidases cleave NAAs exposing the following NAA for sequencing. On-off binding reveals the amino acid sequence in each diverse peptide. Data is securely transferred and analyzed using an intuitive Cloud based software which aligns and translates kinetic signatures to protein identification.
Platinum™ Next-Generation Protein Sequencer
How does next-generation protein sequencing complement existing workflows, especially for researchers primarily focused on genomics or transcriptomics?
The numerous advantage of next-generation protein sequencing lies in its capability to delve deeper into samples, right down to the amino acid level — a facet that sets it other than many other technologies. Whether you use inside a proteomics core or a genomics core, this capability holds substantial value.
Transitioning from genomics to proteomics introduces considerations of accessibility and price, similar to the expenses related to automated evaluation machines and specialized capabilities not typically present in genomics cores.
Fundamentally, genomics laboratories are embracing proteomics technologies to finish the complete narrative. Their journey begins with DNA-level work, extends to RNA and transcriptomics, and ultimately seeks to unveil the intricacies of protein presence and expression. The target is to ascertain a cohesive connection between RNA sequencing outcomes and observations on the proteomic level.
Researchers aim to determine whether proteins align with anticipated results by way of their presence and functionality across all subjects or patients under scrutiny. This pursuit involves investigating potential deviations — be it the absence of expected proteins or the non-uniform performance of identified proteins amongst subjects.
Researchers also strive to uncover protein modifications and diverse proteoforms, further enriching the multiomic exploration. The progression spans from foundational DNA evaluation to transcriptomics and culminates in a comprehensive understanding of functional occurrences on the protein stratum.
What precious insights can researchers gain from the Platinum™ Next-Generation Protein Sequencer?
There are distinct customer segments that derive unique advantages from Platinum™ Next-Generation Protein Sequencer. One group comprises individuals who seek to conduct their research autonomously, thereby exerting control over the research timeline and maintaining ownership from sample collection to eventual publication. For them, this technology fulfills the elemental aspiration of comprehensive research ownership across all stages.
Conversely, those already immersed in proteomics harness the capabilities of the sequencer to delve into the intricacies of data. In cases where samples harbor hypothesized amino acid alterations or discrepancies between responder and non-responder profiles, this tool facilitates the investigation of potential protein modifications, variants or other changes.
Researchers at this point possess hypotheses that they could have pursued using existing methods, yet they aspire to delve even further, pinpointing underlying causes and substantiating their conjectures. The sequencer empowers them to embark on a journey of discovery, enabling the exploration of things they consider causative or significant yet are unable to accurately detect using current methodologies.
How does the Platinum™ Next-Generation Protein Sequencer enhance the capabilities of researchers using immunoassays for protein evaluation?
When utilizing Platinum™ Next-Generation Protein Sequencer together with immunoassays for protein evaluation, a big shift in approach becomes apparent. Within the realm of immunoassays, the standard process involves deliberate selection and targeting of specific proteins, a biased methodology necessitating a transparent predefined objective.
In contrast, the distinct advantage offered by Platinum™ Next-Generation Protein Sequencer lies in its capability for unbiased evaluation. Throughout the domain of proteomics, this technology permits researchers to explore samples without the constraint of predefining their goal. This attribute fundamentally alters the dynamics, rendering the normal necessity of repeatedly procuring or designing specialized reagents obsolete.
Each iteration of a study in the normal immunoassay paradigm necessitates the acquisition or creation of specific reagents tailored to the protein of interest.
In contrast, Platinum™ Next-Generation Protein Sequencer streamlines this process by enabling uniformity in workflow and reagent usage across diverse investigations. This consistency is maintained no matter the particular protein being studied, offering unparalleled efficiency and resource utilization.
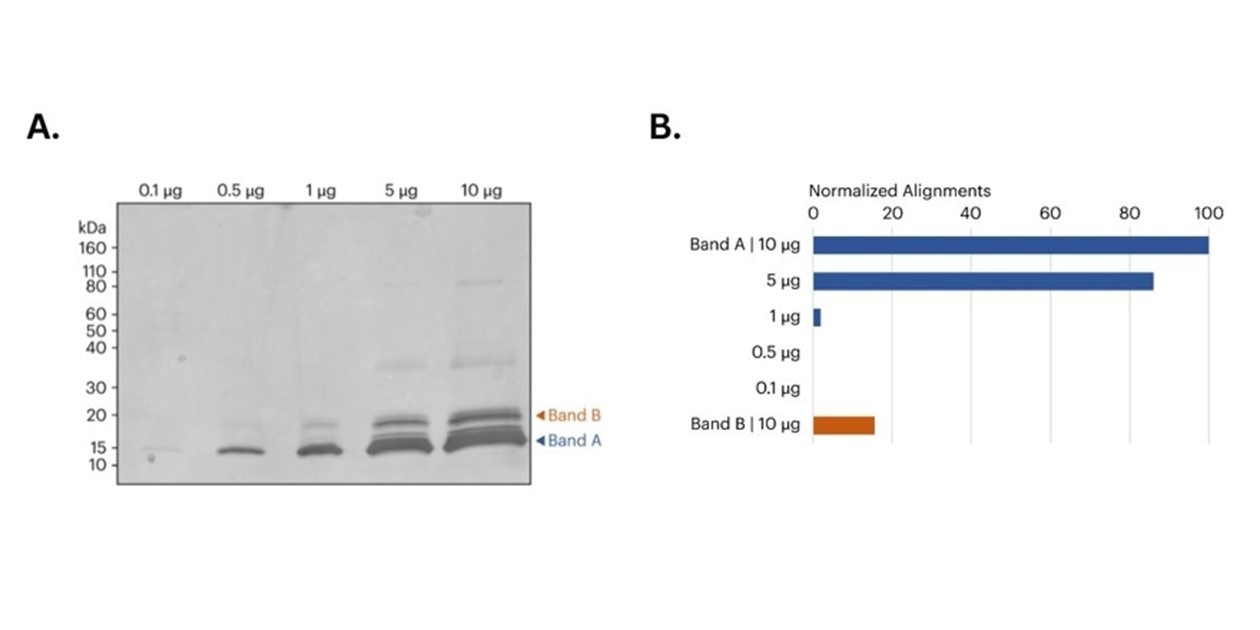
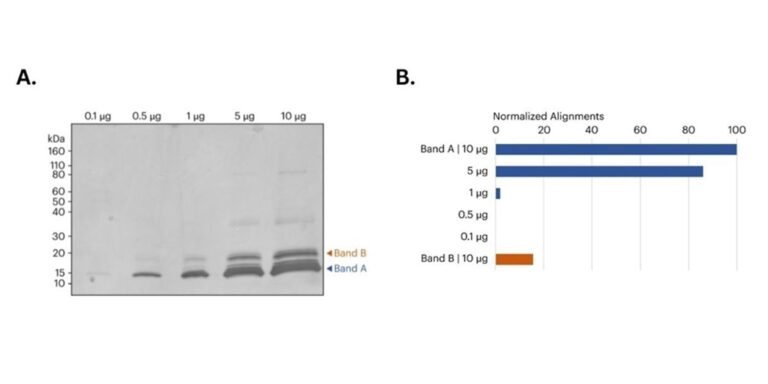
Figure 2. A) image of SDS-PAGE gel of CDNF samples prior to band excision. Orange and blue arrows indicate the approximate location of the bands removed for in-gel digestion and subsequent protein sequencing on Platinum. B) Bar graph shows the relative variety of peptide alignments for every in-gel digested sample. For Band A, each the 5 μg and 10 μg samples produced more aligned CDNF peptides in comparison with the 0.1 μg, 0.5 μg, and 1 μg samples. Moreover, the ten μg Band B sample also produced a big variety of CDNF aligned peptides. All alignments normalized to the overall alignments from the ten μg library.
The inherent advantage of employing an unbiased technology is the capability to not only discover the sought-after protein but in addition uncover additional proteins of interest that might need gone otherwise unnoticed.
This capability is especially crucial when piecing together a comprehensive understanding of protein interactions and dynamics inside a posh biological system.
Are you able to share specific examples where next-generation protein sequencing has significantly advanced proteomics research and improved our understanding of complex biological processes?
A compelling example, detailed in an application note available on our website, stems from a collaborative effort with an educational institution. On this case, the main focus was on individuals who had contracted COVID-19. The aim was to discern variations within the immune responses amongst those infected by different strains like Omicron, Delta, and the unique Alpha strain, thereby unraveling crucial distinctions.
Through this partnership, we managed to unveil subtle alterations, similar to single amino acid changes and the presence/absence of specific peptides. The flexibility to sequence on the amino acid level played a pivotal role. By leveraging this approach, it became feasible to accurately pinpoint whether a person was impacted by Omicron or Delta, effectively enabling a type of surveillance.
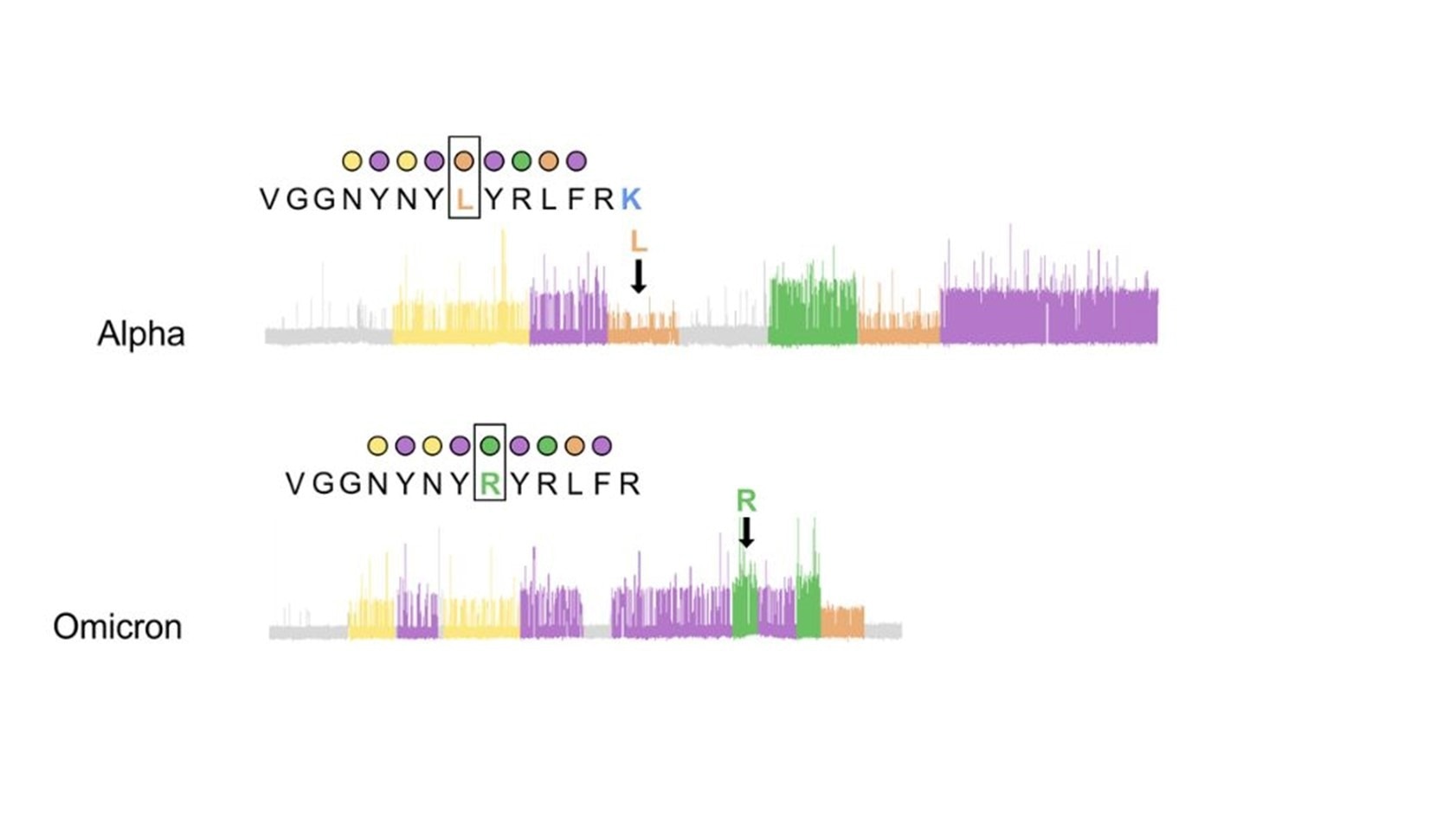
Figure 3. Example protein sequencing traces of SARS-CoV-2 variants for the peptide where the L452R mutation occurs shows that recognizer binding produces clear kinetic differences influenced by the L452R mutation that might be detected with Quantum-Si’s Platinum instrument, effectively differentiating the Alpha variant from Omicron.
The importance lies within the scenario where individuals had encountered COVID-19 however the viral RNA had already dissipated. This method offered a novel avenue to analyze the aftermath of infections and analyze the immune responses that endured.
Essentially, it allowed for the monitoring of people’ immune reactions post-infection. This avenue of research holds particular intrigue because it extends the study timeline well beyond the phase when viral RNA might be sequenced, thereby facilitating the identification of viral strains even after their genetic material has waned.
What benefits does the Platinum sequencer offer over traditional mass spectrometry for identifying proteins and proteoforms?
Regarding protein identification, mass spectrometry excels in scenarios where you might want to process numerous samples in a single go — say, working with 100 or 50 samples concurrently.
Nevertheless, it is vital to notice that not every research endeavor requires such extensive scalability. There are instances when your sample pool is smaller or you’re looking for a swift and streamlined identification process without navigating the complexities related to a high-volume workflow.
Conversely, when the target is to delve deeper into evaluation, scrutinizing protein variants becomes pivotal — encompassing protein modifications and single amino acid substitutions. While certain mass spectrometry methods can address a few of these intricacies, they often entail arduous preparatory stages and other procedural intricacies.
That is where Platinum sequencer distinguishes itself by enabling the execution of those analyses through a more standard workflow on our platform. The distinct advantage emerges as you aim for profound insights, delving into intricate protein details.
Nevertheless, it’s value highlighting that there may be also value in conducting fundamental protein analyses, especially before your research undertakings advance to the size of a whole bunch of samples. In these contexts, Platinum offers a swifter and more accessible approach to protein identification.
How do you see next-generation protein sequencing shaping the longer term of proteomics research and contributing to scientific discoveries on this field?
The numerous impact of next-generation protein sequencing lies in its potential to drive transformative discoveries. An array of instances involving targeted therapeutics underscores the relevance of pinpointing specific proteins.
Upon analyzing clinical data, patterns emerge where individuals exhibit distinct responses, including responders and non-responders, in addition to reoccurrence and non-recurrence cases. Delving into this divergence and elucidating its underpinnings constitutes a point of interest of exploration for varied researchers and publications.
The inspiration of those disparities often pertains to post-translational modifications (PTMs) and a broader spectrum of protein variations, which, while fundamentally sharing protein-level similarity, diverge on the amino acid or PTM level.
This divergence offers a precious avenue for stratification, enabling refined identification and the advancement of targeted treatments. Furthermore, this technology bears the potential for patient stratification, diagnostics, and therapeutic assessments. It could function a disease biomarker or aid in monitoring responses, even detecting minimal residual disease.
Notably, each diagnostic and therapeutic realms stand to realize from the newfound capability to unveil these intricacies. The prevailing scenario of uniform treatment yielding diverse outcomes prompts an unbiased, comprehensive exploration to uncover the underlying aspects.
This understanding holds the promise of novel therapeutic approaches, individualized treatments, and early intervention strategies. Ultimately, the insights achievable through such comprehensive evaluation pave the way in which for long-term advancements in proteomics research, ushering in a recent era of potential breakthroughs.
In what unique ways does the Platinum sequencer allow researchers to review modifications, proteoforms, and rare events that antibodies may not detect?
Platinum sequencer offers several distinct benefits on this regard. Our technology presents a more versatile approach than relying solely on antibodies, which necessitates prior knowledge of the particular change being targeted.
Researchers often face limitations when using antibodies, particularly when coping with subtle modifications inside a protein. The intricacy of those modifications could render it difficult to develop highly specific antibodies tailored to those minute alterations.
Nevertheless, with Platinum next-generation sequencing, this challenge is mitigated. By enzymatically breaking down the protein into peptides and subsequently sequencing them on the amino acid level, our technology empowers researchers to explore without the necessity for specialised reagents.
Which means researchers not must develop recent reagents or precisely know upfront what to go looking for. The sequencer facilitates a broader lens approach, allowing researchers to uncover a spectrum of alterations — be it probing for specific protein modifications or identifying substitutions — without the constraints of traditional antibody-based methods.
 Image Credit: Quantum-Si
Image Credit: Quantum-Si
How has the Platinum™ Next-Generation Protein Sequencer encouraged researchers to adopt a more comprehensive multiomics approach?
Within the early stages of our business launch, we observed a notable trend amongst researchers. Specifically, on the proteomics core laboratory level, there may be a growing enthusiasm for conducting in-depth analyses as a part of their research endeavors.
This enthusiasm arises from the need to meticulously decipher distinctions between diverse study populations. As discussed earlier, our presence at trade shows and similar events, often with a genomic focus, has allowed us to witness an increasing variety of genomics labs aiming to complement their capabilities as well.
These labs have already delved into DNA and RNA sequencing, and a few are even venturing into spatial biology domains.
The introduction of proteomics, the capability to sequence proteins, serves as a precious supplementary tool for his or her holistic exploration. This approach empowers researchers to analyze their areas of interest comprehensively, spanning from the DNA level to the functional dimension of proteins.
About Jeff Hawkins
Jeff Hawkins has over 20 years of experience on the world’s leading life science and diagnostics corporations. Prior to Quantum-Si, Jeff was President and Chief Executive Officer of Truvian Sciences, Inc. where he led the evolution of the company’s benchtop blood testing system from a product concept. Jeff Hawkins holds a B.A. in Chemistry with honors from Concordia University in addition to an MBA from Keller Graduate School of Management.
the company’s benchtop blood testing system from a product concept. Jeff Hawkins holds a B.A. in Chemistry with honors from Concordia University in addition to an MBA from Keller Graduate School of Management.
About Quantum-Si
Quantum-Si was originally founded in 2013 by Jonathan Rothberg, who envisioned a recent frontier where biologists can speed up basic scientific insights and biomedical advances through the ability of next-generation protein sequencing.
Quantum-Si goals to revolutionize proteomics, by bringing single-molecule protein evaluation to each lab, in every single place, to generate a greater understanding of proteins’ role in cell biology and disease. The corporate’s instrument, called Platinum™, is powered by a first-of-its-kind semiconductor chip, and enables researchers through next-generation protein sequencing to know what is actually happening on the protein, peptide or amino acid level to fuel recent discoveries.
Platinum™, is powered by a first-of-its-kind semiconductor chip, and enables researchers through next-generation protein sequencing to know what is actually happening on the protein, peptide or amino acid level to fuel recent discoveries.